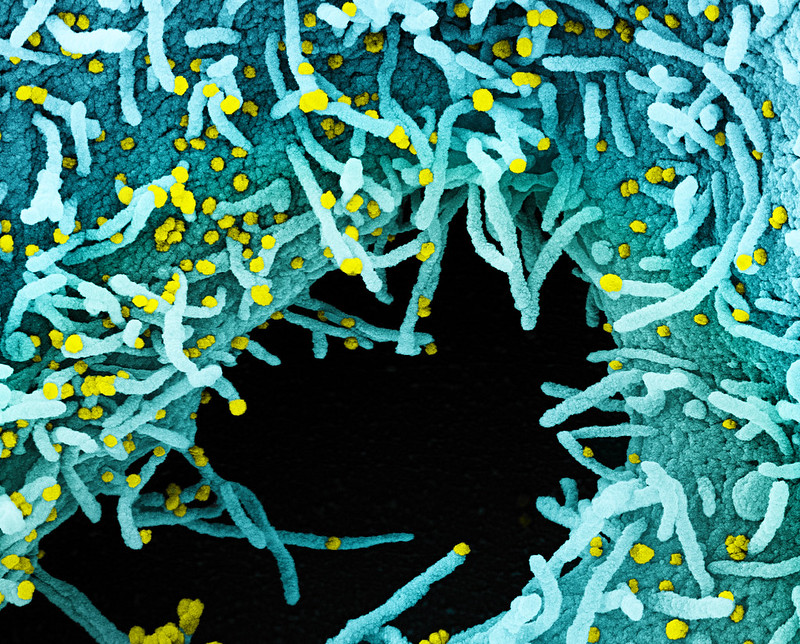
An Israeli national laboratory will begin testing its coronavirus vaccine on human subjects in mid-October, Defense Minister Benny Gantz announced Thursday.
Gantz made the remarks following a visit to the Defense Ministry’s secretive Institute for Biological Research in Ness Ziona, where he was informed of recent “advancements in the development of the vaccine and antibodies for the coronavirus,” his office said.
According to the defense minister, initial tests of the vaccine have been promising, allowing for human trials....
An Israeli national laboratory will begin testing its coronavirus vaccine on human subjects in mid-October, Defense Minister Benny Gantz announced Thursday.
Gantz made the remarks following a visit to the Defense Ministry’s secretive Institute for Biological Research in Ness Ziona, where he was informed of recent “advancements in the development of the vaccine and antibodies for the coronavirus,” his office said.
According to the defense minister, initial tests of the vaccine have been promising, allowing for human trials.
“We should begin tests on people after the Tishrei holidays,” Gantz said, referring to the Hebrew month in which the Jewish High Holidays take place, the last of which ends on October 10. “This will be done in coordination with the Health Ministry and according to the protocols needed in terms of medical safety.”
The director of the Institute for Biological Research, Prof. Shmuel Shapira, said he believed that the vaccine would be successful....
Last month, Channel 12 news reported that the institute had made significant progress on the vaccine, achieving nearly 100 percent efficacy in animals.
The vaccine under development is on par in effectiveness with a vaccine being developed by US biotechnology company Moderna, according to the TV report.
In June, Israel signed a deal with Moderna for the potential purchase of its coronavirus vaccine if it proves effective.
Unlike vaccines developed abroad, the domestic vaccine will first be delivered to Israeli citizens, it added. If successful, it was expected to provide protection against the disease with a single dose.
Though it had not started human trials, the institute was preparing to manufacture 10 to 15 million doses, report said. In June, the institute announced it had completed successful coronavirus vaccine trials on rodents


Recent Comments